In recent years, Ozempic, a medication primarily used for managing type 2 diabetes, has gained
significant attention not only for its medical benefits but also for a less favorable reason. Amidst its growing popularity, there has been a notable increase in legal actions, commonly referred to as Ozempic lawsuits, highlighting concerns beyond its intended health benefits.
This article aims to discuss the intricate details of these lawsuits, unraveling the reasons behind this surge in legal challenges. By exploring various facets of these cases, we seek to provide a comprehensive understanding of the situation, balancing the medical advantages of Ozempic with the legal controversies it currently faces.
What is Ozempic?
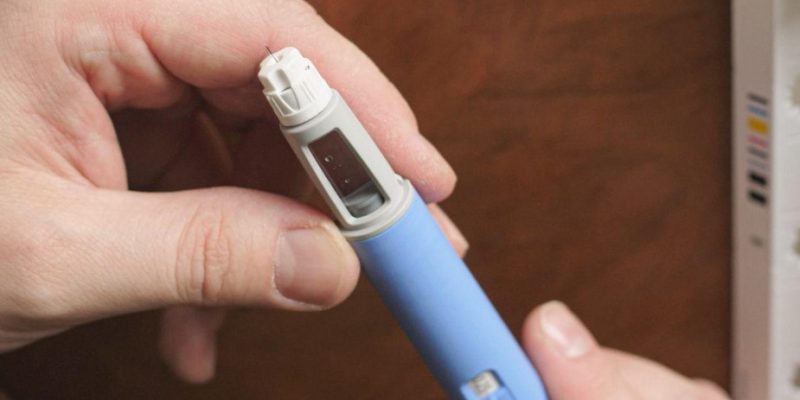
Ozempic, a name that has become increasingly familiar in the healthcare world, is a prescription medication primarily designed to treat type 2 diabetes. Its main active ingredient, semaglutide, works by mimicking a hormone called GLP-1, which is crucial in regulating blood sugar levels.
This mechanism not only aids in controlling blood glucose but also contributes to weight loss, which is a beneficial side effect for many patients. Approved by the FDA in 2017, Ozempic has since been a subject of interest and scrutiny within the medical community. Its journey from regulatory approval to widespread clinical use reflects a complex balance between therapeutic innovation and the need for ongoing safety monitoring.
The Benefits of Ozempic
Ozempic has emerged as a beacon of hope for many grappling with type 2 diabetes. Its primary benefit lies in managing blood sugar levels effectively, a crucial aspect of diabetes care. Beyond this, Ozempic has been observed to aid in weight loss, a common challenge for those with diabetes.
This unintended yet welcome effect has sparked interest in its off-label use for weight management. Numerous patient success stories further underscore its effectiveness. Individuals have reported not only improved diabetic control but also significant lifestyle changes due to weight loss and better overall health.
These positive outcomes paint a picture of Ozempic as a multifaceted medication offering more than just diabetes management.
The Emergence of Ozempic Lawsuits
Ozempic, known for its role in diabetes management and weight loss, has recently become the subject of numerous lawsuits. As of March 2024, 58 personal injury lawsuits were filed for conditions like gastroparesis, ileus, and intestinal blockage or obstruction, centralized in federal multidistrict litigation in the Eastern District of Pennsylvania (MDL 3094). These lawsuits primarily allege that Ozempic and other GLP-1 RAS class drugs caused severe gastrointestinal injuries.
A critical case in this series is Jaclyn Bjorklund, who filed one of the first lawsuits, including claims against Ozempic and Mounjaro, a similar drug. In response to these emerging concerns, the FDA added warnings for intestinal blockage/obstruction and ileus to Ozempic’s label in September 2023.
Despite these legal challenges, no global settlements or jury trials have existed. The plaintiffs in these cases seek compensation for severe health issues like gastroparesis, a condition that slows or stops food movement from the stomach to the small intestine, leading to various complications.
Reasons Behind the Lawsuits
The lawsuits against Ozempic primarily stem from potential side effects and adverse reactions associated with the drug.
- Gastroparesis: A condition causing delayed stomach emptying, leading to nausea and vomiting.
- Intestinal blockage or obstruction: Difficulty in food moving through the intestines.
- Ileus: A condition where the intestines stop working properly.
- Increased risk of gallbladder disease.
- Possible connection to suicidal ideation.
Plaintiffs in the lawsuits argue that Novo Nordisk, the manufacturer of Ozempic, failed to provide adequate warnings about these severe side effects. They claim that the company either concealed or downplayed the risks, making patients unaware of the potential dangers.
The lawsuits have brought attention to the analysis of clinical trials and post-marketing reports of Ozempic. Critics suggest that these reports indicate a higher incidence of severe side effects than initially disclosed, raising questions about the thoroughness and transparency of the drug’s safety evaluations.
The Impact of Lawsuits on Patients and Healthcare
The unfolding lawsuits against Ozempic have created a ripple effect, influencing both current and potential users of the drug. Many are cautiously approaching Ozempic, weighing its benefits against the reported risks. This shift in perception is also reflected in the healthcare community.
Healthcare professionals and endocrinologists are increasingly vigilant, closely monitoring their patients on Ozempic for adverse effects and re-evaluating its prescription guidelines. Moreover, these lawsuits have prompted regulatory agencies to take a more active role.
Agencies like the FDA are scrutinizing the drug’s safety profile more intensely, leading to updated warnings and a re-assessment of its overall risk-benefit balance. This heightened oversight ensures patient safety while maintaining access to effective diabetes management options.
Legal Perspectives
Legal experts view the Ozempic lawsuits as a significant development in pharmaceutical litigation. They highlight the complexity of these cases, given the drug’s widespread use and the severity of the alleged side effects.
These lawsuits are similar to past legal actions against other pharmaceutical companies, where issues of inadequate warnings and adverse health effects were central. Experts suggest that the Ozempic lawsuits could lead to substantial settlements or judgments, mirroring outcomes seen in other high-profile drug litigation cases.
The potential outcomes of these lawsuits carry broad implications, including stricter regulatory measures, more rigorous drug testing protocols, and a possible shift in how pharmaceutical companies market and disclose information about their products. These actions could set precedents influencing future pharmaceutical litigation and patient safety standards.
Conclusion
The Ozempic lawsuits have cast a spotlight on the drug’s potential side effects and raised questions about the adequacy of warnings provided to users. These legal challenges reflect a growing concern over patient safety and the responsibility of pharmaceutical companies to disclose drug risks.
The broader implications for the industry include a potential shift towards more stringent safety regulations and transparent marketing practices. As for the future of Ozempic and similar medications, these developments may lead to more cautious prescribing practices and heightened scrutiny in drug approval processes, ultimately aiming to balance patient health benefits with safety.